Search results
Search for "reactive intermediate" in Full Text gives 60 result(s) in Beilstein Journal of Organic Chemistry.
Photoinduced in situ generation of DNA-targeting ligands: DNA-binding and DNA-photodamaging properties of benzo[c]quinolizinium ions
- Julika Schlosser,
- Olga Fedorova,
- Yuri Fedorov and
- Heiko Ihmels
Beilstein J. Org. Chem. 2024, 20, 101–117, doi:10.3762/bjoc.20.11
- species (ROS), such peroxyl, alkoxy and hydroxyl radicals, or carbon-centered radicals, which subsequently induce DNA strand cleavage. In the type-II mechanism, a triplet-excited photosensitizer reacts with molecular oxygen to give highly reactive singlet oxygen, 1O2, as reactive intermediate, which in
- reactive intermediate 1O2 is known to induce DNA damage [78][88]. Nevertheless, these lesions, namely DNA base oxidations, often require alkaline treatment to lead to a strand cleavage, so that the DNA damage remained mainly unnoticed in the employed assay. At the same time, it has been reported that 1O2

Graphical Abstract

Scheme 1: Photoinduced formation of benzo[c]quinolizinium and its interaction with DNA upon intercalation.

Scheme 2: Synthesis of styrylpyridine derivatives 2a–g. Conditions: i: piperidine, MeOH, reflux (2a,c), ii: C...

Figure 1: Absorption spectra of styrylpyridine derivatives 2a (black), 2b (red), 2c (blue), 2d (green), 2e (m...

Figure 2: Changes of the absorption spectra during the irradiation of 2a in MeCN for 16 min (A), 2b in MeCN f...

Figure 3: Changes of the absorption spectra during the irradiation of 2c for 13 min (A), 2d for 12 min (B), 2e...

Scheme 3: Photoinduced formation of styrylpyridine derivatives 2b–g to the benzo[c]quinolizinium ions 3b–g (y...

Figure 4: Photometric titration of ct DNA to 3c (A) 3e (B) 3f (C) and 3g (D) (c = 20 µM) in Na phosphate buff...

Figure 5: Fluorimetric titration of ct DNA to 3c (A), 3e (B), 3f (C), and 3g (D) (c = 20 µM) in Na phosphate ...

Figure 6: CD (A) and LD (B) spectra of 3f and ct DNA (cDNA = 20 µM) in Na phosphate buffer (pH 7.0, T = 20 °C...

Figure 7: Changes of the absorption (A) and CD (B) spectra during the irradiation of 2e (1) and 2f (2) (c = 2...

Scheme 4: Proposed mechanisms for the photoinduced DNA damage initiated by photoexcitation of benzoquinolizin...
Photoredox catalysis harvesting multiple photon or electrochemical energies
- Mattia Lepori,
- Simon Schmid and
- Joshua P. Barham
Beilstein J. Org. Chem. 2023, 19, 1055–1145, doi:10.3762/bjoc.19.81
- neutral PDI and forms the aryl halide’s radical anion, which then undergoes C(sp2)–X bond fission to afford the aryl radical as a reactive intermediate. The aryl radical then either reacts via hydrogen atom transfer (HAT) with solvent molecules or Et3N•+ in an overall dehalogenation to furnish product 2

Graphical Abstract

Figure 1: Oxidative and reductive activations of organic compounds harvesting photoredox catalysis.

Figure 2: General catalytic cycles of radical ion conPET (left) and radical ion e-PRC (right).

Figure 3: “Beginner’s guide”: comparison between advantages, capacities, and prospectives of conPET and PEC.

Figure 4: A) conPET reductive dehalogenation of aryl halides with PDI. B) Reductive C–H arylation with pyrrol...

Figure 5: A) Chromoselective mono- and disubstitution or polybrominated pyrimidines with pyrroles. B) Sequent...

Figure 6: A) Synthesis of pyrrolo[1,2-a]quinolines. B) Synthesis of ullazines.

Figure 7: A) Reductive phosphorylation of aryl halides via conPET. B) Selected examples from the substrate sc...

Figure 8: A) Reductive dehalogenation of aryl halides via conPET and selected examples from the substrate sco...

Figure 9: A) Reductive C–H arylation of aryl halides via conPET (top) and selected examples from the substrat...

Figure 10: A) Reductive hydrodehalogenation of aryl halides with Mes-Acr-BF4. B) Selected examples from the su...

Figure 11: A) Reductive hydrodechlorination of aryl chlorides with 4-DPAIPN. B) Proposed formation of CO2•−. C...

Figure 12: A) Reductive conPET borylation with 3CzEPAIPN (top) and selected examples from the substrate scope ...

Figure 13: Scale-up of conPET phosphorylation with 3CzEPAIPN.

Figure 14: A) Borylation of 1d. B) Characteristics and structure of PC1 with green and red parts showing the l...

Figure 15: A) Reductive C–H arylation scope with polysulfide conPET (top) and selected examples from the subst...

Figure 16: Scale-up of A) C–H arylation and B) dehaloborylation with polysulfide photocatalysis in continuous-...

Figure 17: A) Formation of [Ir1]0 and [Ir2]0 upon PET between [Ir1]+ and Et3N. B) Mechanism of multi-photon ta...

Figure 18: A) Reductive hydrodehalogenation of aryl halides via multi-photon tandem photocatalysis. B) Selecte...

Figure 19: A) Carbonylative amidation of aryl halides in continuous flow. B) Selected examples from the substr...

Figure 20: A) General scheme for reductive (RQ) and oxidative quenching (OQ) protocols using [FeIII(btz)3](PF6)...

Figure 21: A) Carbonylative amidation of alkyl iodides with [IrIII(ppy)2(dtbbpy)]PF6. B) Selected examples fro...

Figure 22: A) Carboxylative C–N bond cleavage in cyclic amines. B) Selected examples from the substrate scope....

Figure 23: A) Formal reduction of alkenes to alkanes via transfer hydrogenation. B) Selected examples from the...

Figure 24: A) Birch-type reduction of benzenes with PMP-BPI. B) Selected examples from the substrate scope (sc...

Figure 25: Proposed mechanism of the OH− mediated conPET Birch-type reduction of benzene via generation of sol...

Figure 26: Reductive detosylation of N-tosylated amides with Mes-Acr-BF4. B) Selected examples from the substr...

Figure 27: A) Reductive detosylation of N-tosyl amides by dual PRC. B) Selected examples from the substrate sc...

Figure 28: A) Mechanism of the dual PRC based on PET between [Cu(dap)2]+ and DCA. B) Mechanism of the dual PRC...

Figure 29: A) N–O bond cleavage in Weinreb amides with anthracene. B) N–O bond cleavage in Weinreb amides rely...

Figure 30: A) Pentafluorosulfanylation and fluoride elimination. B) Mechanism of the pentafluorosulfanylation ...

Figure 31: A) α-Alkoxypentafluorosulfanylation (top) and selected examples from the substrate scope (bottom). ...

Figure 32: A) Oxidative amination of arenes with azoles catalyzed by N-Ph PTZ. B) Selected examples from the s...

Figure 33: A) C(sp3)–H bond activation by HAT via chloride oxidation by *N-Ph PTZ•+. B) Proposed mechanism for...

Figure 34: A) Recycling e-PRC C–H azolation of electron-rich arenes with pyrazoles using Mes-Acr+ as a photoca...

Figure 35: A) Radical ion e-PRC direct oxidation of unactivated arenes using TAC+ as an electro-activated phot...

Figure 36: A) Radical ion e-PRC direct oxidation of unactivated arenes using TPA as an electro-activated photo...

Figure 37: Proposed mechanism (top) and mode of preassembly (bottom).

Figure 38: A) Possible preassemblies of reactive (left) vs unreactive (right) arenes. B) Calculated spin densi...

Figure 39: A) Recycling e-PRC C(sp2 )–H acetoxylation of arenes using DDQ as a photocatalyst. B) Proposed cata...

Figure 40: Gram scale hydroxylation of benzene in a recirculated flow setup.

Figure 41: A) Radical ion e-PRC vicinal diamination of alkylarenes using TAC+ as an electro-activated photocat...

Figure 42: A) Sequential oxygenation of multiple adjacent C–H bonds under radical ion e-PRC using TAC+ as an e...

Figure 43: A) Enantioselective recycling e-PRC cyanation of benzylic C–H bonds using ADQS as photocatalyst. B)...

Figure 44: Proposed tandem mechanism by Xu and co-workers.

Figure 45: A) Enantioselective recycling e-PRC decarboxylative cyanation using Cu(acac)2, Ce(OTf)3 and a box l...

Figure 46: A) Enantioselective recycling e-PRC benzylic cyanation using Cu(MeCN)4BF4, box ligand and anthraqui...

Figure 47: A) Radical ion e-PRC acetoxyhydroxylation of aryl olefins using TAC+ as an electro-activated photoc...

Figure 48: Selected examples from the substrate scope.

Figure 49: Photoelectrochemical acetoxyhydroxylation in a recirculated flow setup.

Figure 50: A) Radical ion e-PRC aminooxygenation of aryl olefins using TAC+ as an electro-activated photocatal...

Figure 51: A) Recycling e-PRC C–H alkylation of heteroarenes with organic trifluoroborates using Mes-Acr+ as p...

Figure 52: A) Recycling e-PRC decarboxylative C–H alkylation of heteroarenes using CeCl3·7H2O as catalyst. B) ...

Figure 53: A) Recycling e-PRC decarboxylative C–H alkylation of heteroarenes using Fe(NH4)2(SO4)2·6H2O as cata...

Figure 54: A) Recycling e-PRC C–H alkylation of heteroarenes with alkyl oxalates and 4CzIPN as photocatalyst. ...

Figure 55: A) Recycling e-PRC decarboxylative C–H carbamoylation of heteroarenes using 4CzIPN as photocatalyst...

Figure 56: A) Photoelectrochemical HAT-mediated hydrocarbon activation via the chlorine radical. B) Proposed m...

Figure 57: A) Selected examples from the substrate scope. B) Gram and decagram scale semi-continuous flow PEC ...

Figure 58: A) Photoelectrochemical HAT-mediated dehydrogenative coupling of benzothiazoles with aliphatic C–H ...

Figure 59: A) Photoelectrochemical HAT activation of ethers using electro-activated TAC+ as photocatalyst. B) ...

Figure 60: Selected examples from the substrate scope.

Figure 61: A) Photoelectrochemical HAT-mediated synthesis of alkylated benzimidazo-fused isoquinolinones using...

Figure 62: A) Decoupled photoelectrochemical cerium-catalyzed oxydichlorination of alkynes using CeCl3 as cata...

Figure 63: Proposed decoupled photoelectrochemical mechanism.

Figure 64: A) Decoupled photoelectrochemical ring-opening bromination of tertiary cycloalkanols using MgBr2 as...

Figure 65: A) Recycling e-PRC ring-opening functionalization of cycloalkanols using CeCl3 as catalyst. B) Prop...

Figure 66: Selected examples from the substrate scope of the PEC ring-opening functionalization.

Figure 67: A) Radical ion e-PRC reduction of chloro- and bromoarenes using DCA as catalyst and various accepto...

Figure 68: A) Screening of different phthalimide derivatives as catalyst for the e-PRC reduction of aryl halid...

Figure 69: Screening of different organic catalysts for the e-PRC reduction of trialkylanilium salts.

Figure 70: A) e-PRC reduction of phosphonated phenols and anilinium salts. B) Selected examples from the subst...

Figure 71: A) ConPET and e-PRC reduction of 4-bromobenzonitrile using a naphthalene diimide (NDI) precatalyst ...

Figure 72: A) Radical ion e-PRC reduction of phosphinated aliphatic alcohols with n-BuO-NpMI as catalyst. B) C...

Figure 73: Selected examples from the substrate scope.

Figure 74: A) Recycling e-PRC reductive dimerization of benzylic chlorides using a [Cu2] catalyst. B) Proposed...

Figure 75: A) Decoupled photoelectrochemical C–H alkylation of heteroarenes through deamination of Katritzky s...

Figure 76: Proposed mechanism by Chen and co-workers.
Copper-catalyzed N-arylation of amines with aryliodonium ylides in water
- Kasturi U. Nabar,
- Bhalchandra M. Bhanage and
- Sudam G. Dawande
Beilstein J. Org. Chem. 2023, 19, 1008–1014, doi:10.3762/bjoc.19.76
- generation of carbene as a reactive intermediate [36][37]. Also, spirocyclic iodonium ylides have been used for radiolabeling techniques [38]. In 2013, Shibata’s research group reported a novel trifluoromethanesulfonyl iodonium ylide for trifluoromethylthiolation of enamines, indoles, and ketoesters

Graphical Abstract

Figure 1: Representative examples of N-arylamines.

Scheme 1: N-Arylation of amines with hypervalent iodine reagents.

Scheme 2: N-Arylation of primary amines with iodonium ylide. Reaction conditions: 0.2 mmol aniline 1, 0.24 mm...

Scheme 3: N-Arylation of secondary amines with iodonium ylide.
Iron-catalyzed domino coupling reactions of π-systems
- Austin Pounder and
- William Tam
Beilstein J. Org. Chem. 2021, 17, 2848–2893, doi:10.3762/bjoc.17.196
- is the termination of the reaction through the trapping of the reactive intermediate. Organoiron complexes have been shown to undergo electrophilic trapping with external species or proceed through cross-coupling eventually undergoing reductive elimination. Radical addition will typically conclude

Graphical Abstract

Figure 1: Price comparison among iron and other transition metals used in catalysis.

Scheme 1: Typical modes of C–C bond formation.

Scheme 2: The components of an iron-catalyzed domino reaction.

Scheme 3: Iron-catalyzed tandem cyclization and cross-coupling reactions of iodoalkanes 1 with aryl Grignard ...

Scheme 4: Three component iron-catalyzed dicarbofunctionalization of vinyl cyclopropanes 14.

Scheme 5: Three-component iron-catalyzed dicarbofunctionalization of alkenes 21.

Scheme 6: Double carbomagnesiation of internal alkynes 31 with alkyl Grignard reagents 32.

Scheme 7: Iron-catalyzed cycloisomerization/cross-coupling of enyne derivatives 35 with alkyl Grignard reagen...

Scheme 8: Iron-catalyzed spirocyclization/cross-coupling cascade.

Scheme 9: Iron-catalyzed alkenylboration of alkenes 50.

Scheme 10: N-Alkyl–N-aryl acrylamide 60 CDC cyclization with C(sp3)–H bonds adjacent to a heteroatom.

Scheme 11: 1,2-Carboacylation of activated alkenes 60 with aldehydes 65 and alcohols 67.

Scheme 12: Iron-catalyzed dicarbonylation of activated alkenes 68 with alcohols 67.

Scheme 13: Iron-catalyzed cyanoalkylation/radical dearomatization of acrylamides 75.

Scheme 14: Synergistic photoredox/iron-catalyzed 1,2-dialkylation of alkenes 82 with common alkanes 83 and 1,3...

Scheme 15: Iron-catalyzed oxidative coupling/cyclization of phenol derivatives 86 and alkenes 87.

Scheme 16: Iron-catalyzed carbosulfonylation of activated alkenes 60.

Scheme 17: Iron-catalyzed oxidative spirocyclization of N-arylpropiolamides 91 with silanes 92 and tert-butyl ...

Scheme 18: Iron-catalyzed free radical cascade difunctionalization of unsaturated benzamides 94 with silanes 92...

Scheme 19: Iron-catalyzed cyclization of olefinic dicarbonyl compounds 97 and 100 with C(sp3)–H bonds.

Scheme 20: Radical difunctionalization of o-vinylanilides 102 with ketones and esters 103.

Scheme 21: Dehydrogenative 1,2-carboamination of alkenes 82 with alkyl nitriles 76 and amines 105.

Scheme 22: Iron-catalyzed intermolecular 1,2-difunctionalization of conjugated alkenes 107 with silanes 92 and...

Scheme 23: Four-component radical difunctionalization of chemically distinct alkenes 114/115 with aldehydes 65...

Scheme 24: Iron-catalyzed carbocarbonylation of activated alkenes 60 with carbazates 117.

Scheme 25: Iron-catalyzed radical 6-endo cyclization of dienes 119 with carbazates 117.

Scheme 26: Iron-catalyzed decarboxylative synthesis of functionalized oxindoles 130 with tert-butyl peresters ...

Scheme 27: Iron‑catalyzed decarboxylative alkylation/cyclization of cinnamamides 131/134.

Scheme 28: Iron-catalyzed carbochloromethylation of activated alkenes 60.

Scheme 29: Iron-catalyzed trifluoromethylation of dienes 142.

Scheme 30: Iron-catalyzed, silver-mediated arylalkylation of conjugated alkenes 115.

Scheme 31: Iron-catalyzed three-component carboazidation of conjugated alkenes 115 with alkanes 101/139b and t...

Scheme 32: Iron-catalyzed carboazidation of alkenes 82 and alkynes 160 with iodoalkanes 20 and trimethylsilyl ...

Scheme 33: Iron-catalyzed asymmetric carboazidation of styrene derivatives 115.

Scheme 34: Iron-catalyzed carboamination of conjugated alkenes 115 with alkyl diacyl peroxides 163 and acetoni...

Scheme 35: Iron-catalyzed carboamination using oxime esters 165 and arenes 166.

Scheme 36: Iron-catalyzed iminyl radical-triggered [5 + 2] and [5 + 1] annulation reactions with oxime esters ...

Scheme 37: Iron-catalyzed decarboxylative alkyl etherification of alkenes 108 with alcohols 67 and aliphatic a...

Scheme 38: Iron-catalyzed inter-/intramolecular alkylative cyclization of carboxylic acid and alcohol-tethered...

Scheme 39: Iron-catalyzed intermolecular trifluoromethyl-acyloxylation of styrene derivatives 115.

Scheme 40: Iron-catalyzed carboiodination of terminal alkenes and alkynes 180.

Scheme 41: Copper/iron-cocatalyzed cascade perfluoroalkylation/cyclization of 1,6-enynes 183/185.

Scheme 42: Iron-catalyzed stereoselective carbosilylation of internal alkynes 187.

Scheme 43: Synergistic photoredox/iron catalyzed difluoroalkylation–thiolation of alkenes 82.

Scheme 44: Iron-catalyzed three-component aminoazidation of alkenes 82.

Scheme 45: Iron-catalyzed intra-/intermolecular aminoazidation of alkenes 194.

Scheme 46: Stereoselective iron-catalyzed oxyazidation of enamides 196 using hypervalent iodine reagents 197.

Scheme 47: Iron-catalyzed aminooxygenation for the synthesis of unprotected amino alcohols 200.

Scheme 48: Iron-catalyzed intramolecular aminofluorination of alkenes 209.

Scheme 49: Iron-catalyzed intramolecular aminochlorination and aminobromination of alkenes 209.

Scheme 50: Iron-catalyzed intermolecular aminofluorination of alkenes 82.

Scheme 51: Iron-catalyzed aminochlorination of alkenes 82.

Scheme 52: Iron-catalyzed phosphinoylazidation of alkenes 108.

Scheme 53: Synergistic photoredox/iron-catalyzed three-component aminoselenation of trisubstituted alkenes 82.
Visible-light-mediated copper photocatalysis for organic syntheses
- Yajing Zhang,
- Qian Wang,
- Zongsheng Yan,
- Donglai Ma and
- Yuguang Zheng
Beilstein J. Org. Chem. 2021, 17, 2520–2542, doi:10.3762/bjoc.17.169
- – is stabilized by the copper complex. The alkyl radical reacts with LnCuII-SO2Cl to deliver the target product 5. A mechanistic study demonstrated that [Cu(dap)2]Cl can coordinate with the reactive intermediate SO2Cl and suppresses the extrusion of SO2. Thus, [Cu(dap)2]Cl achieves a unique

Graphical Abstract

Scheme 1: Photoredox catalysis mechanism of [Ru(bpy)3]2+.

Scheme 2: Photoredox catalysis mechanism of CuI.

Scheme 3: Ligands and CuI complexes.

Scheme 4: Mechanism of CuI-based photocatalysis.

Scheme 5: Mechanisms of CuI–substrate complexes.

Scheme 6: Mechanism of CuII-base photocatalysis.

Scheme 7: Olefinic C–H functionalization and allylic alkylation.

Scheme 8: Cross-coupling of unactivated alkenes and CF3SO2Cl.

Scheme 9: Chlorosulfonylation/cyanofluoroalkylation of alkenes.

Scheme 10: Hydroamination of alkenes.

Scheme 11: Cross-coupling reaction of alkenes, alkyl halides with nucleophiles.

Scheme 12: Cross-coupling of alkenes with oxime esters.

Scheme 13: Oxo-azidation of vinyl arenes.

Scheme 14: Azidation/difunctionalization of vinyl arenes.

Scheme 15: Photoinitiated copper-catalyzed Sonogashira reaction.

Scheme 16: Alkyne functionalization reactions.

Scheme 17: Alkynylation of dihydroquinoxalin-2-ones with terminal alkynes.

Scheme 18: Decarboxylative alkynylation of redox-active esters.

Scheme 19: Aerobic oxidative C(sp)–S coupling reaction.

Scheme 20: Copper-catalyzed alkylation of carbazoles with alkyl halides.

Scheme 21: C–N coupling of organic halides with amides and aliphatic amines.

Scheme 22: Copper-catalyzed C–X (N, S, O) bond formation reactions.

Scheme 23: Arylation of C(sp2)–H bonds of azoles.

Scheme 24: C–C cross-coupling of aryl halides and heteroarenes.

Scheme 25: Benzylic or α-amino C–H functionalization.

Scheme 26: α-Amino C–H functionalization of aromatic amines.

Scheme 27: C–H functionalization of aromatic amines.

Scheme 28: α-Amino-C–H and alkyl C–H functionalization reactions.

Scheme 29: Other copper-photocatalyzed reactions.

Scheme 30: Cross-coupling of oxime esters with phenols or amines.

Scheme 31: Alkylation of heteroarene N-oxides.
Photoredox catalysis in nickel-catalyzed C–H functionalization
- Lusina Mantry,
- Rajaram Maayuri,
- Vikash Kumar and
- Parthasarathy Gandeepan
Beilstein J. Org. Chem. 2021, 17, 2209–2259, doi:10.3762/bjoc.17.143
- involves an energy-transfer pathway generating an electronically excited nickel complex as a key reactive intermediate (Figure 11). Photochemical nickel catalysis was used to synthesize 1,1-diarylalkanes 39 from unactivated alkyl bromides 38 and aryl bromides 3 through a reductive migratory cross-coupling

Graphical Abstract

Scheme 1: Nickel-catalyzed cross-coupling versus C‒H activation.

Figure 1: Oxidative and reductive quenching cycles of a photocatalyst. [PC] = photocatalyst, A = acceptor, D ...

Scheme 2: Photoredox nickel-catalyzed C(sp3)–H arylation of dimethylaniline (1a).

Scheme 3: Photoredox nickel-catalyzed arylation of α-amino, α-oxy and benzylic C(sp3)‒H bonds with aryl bromi...

Figure 2: Proposed catalytic cycle for the photoredox-mediated HAT and nickel catalysis enabled C(sp3)‒H aryl...

Scheme 4: Photoredox arylation of α-amino C(sp3)‒H bonds with aryl iodides.

Figure 3: Proposed mechanism for photoredox nickel-catalyzed α-amino C‒H arylation with aryl iodides.

Scheme 5: Nickel-catalyzed α-oxy C(sp3)−H arylation of cyclic and acyclic ethers.

Figure 4: Proposed catalytic cycle for the C(sp3)−H arylation of cyclic and acyclic ethers.

Scheme 6: Photochemical nickel-catalyzed C–H arylation of ethers.

Figure 5: Proposed catalytic cycle for the nickel-catalyzed arylation of ethers with aryl bromides.

Scheme 7: Nickel-catalyzed α-amino C(sp3)‒H arylation with aryl tosylates.

Scheme 8: Arylation of α-amino C(sp3)‒H bonds by in situ generated aryl tosylates from phenols.

Scheme 9: Formylation of aryl chlorides through redox-neutral 2-functionalization of 1,3-dioxolane (13).

Scheme 10: Photochemical C(sp3)–H arylation via a dual polyoxometalate HAT and nickel catalytic manifold.

Figure 6: Proposed mechanism for C(sp3)–H arylation through dual polyoxometalate HAT and nickel catalytic man...

Scheme 11: Photochemical nickel-catalyzed α-hydroxy C‒H arylation.

Scheme 12: Photochemical synthesis of fluoxetine (21).

Scheme 13: Photochemical nickel-catalyzed allylic C(sp3)‒H arylation with aryl bromides.

Figure 7: Proposed mechanism for the photochemical nickel-catalyzed allylic C(sp3)‒H arylation with aryl brom...

Scheme 14: Photochemical C(sp3)‒H arylation by the synergy of ketone HAT catalysis and nickel catalysis.

Figure 8: Proposed mechanism for photochemical C(sp3)‒H arylation by the synergy of ketone HAT catalysis and ...

Scheme 15: Benzophenone- and nickel-catalyzed photoredox benzylic C–H arylation.

Scheme 16: Benzaldehyde- and nickel-catalyzed photoredox C(sp3)–H arylation.

Scheme 17: Photoredox and nickel-catalyzed enantioselective benzylic C–H arylation.

Figure 9: Proposed mechanism for the photoredox and nickel-catalyzed enantioselective benzylic C–H arylation.

Scheme 18: Photoredox nickel-catalyzed α-(sp3)‒H arylation of secondary benzamides with aryl bromides.

Scheme 19: Enantioselective sp3 α-arylation of benzamides.

Scheme 20: Nickel-catalyzed decarboxylative vinylation/C‒H arylation of cyclic oxalates.

Figure 10: Proposed mechanism for the nickel-catalyzed decarboxylative vinylation/C‒H arylation of cyclic oxal...

Scheme 21: C(sp3)−H arylation of bioactive molecules using mpg-CN photocatalysis and nickel catalysis.

Figure 11: Proposed mechanism for the mpg-CN/nickel photocatalytic C(sp3)–H arylation.

Scheme 22: Nickel-catalyzed synthesis of 1,1-diarylalkanes from alkyl bromides and aryl bromides.

Figure 12: Proposed mechanism for photoredox nickel-catalyzed C(sp3)–H alkylation via polarity-matched HAT.

Scheme 23: Photoredox nickel-catalyzed C(sp3)‒H alkylation via polarity-matched HAT.

Scheme 24: Benzaldehyde- and nickel-catalyzed photoredox C(sp3)‒H alkylation of ethers.

Scheme 25: Benzaldehyde- and nickel-catalyzed photoredox C(sp3)‒H alkylation of amides and thioethers.

Scheme 26: Photoredox and nickel-catalyzed C(sp3)‒H alkylation of benzamides with alkyl bromides.

Scheme 27: CzIPN and nickel-catalyzed C(sp3)‒H alkylation of ethers with alkyl bromides.

Figure 13: Proposed mechanism for the CzIPN and nickel-catalyzed C(sp3)‒H alkylation of ethers.

Scheme 28: Nickel/photoredox-catalyzed methylation of (hetero)aryl chlorides and acid chlorides using trimethy...

Figure 14: Proposed catalytic cycle for the nickel/photoredox-catalyzed methylation of (hetero)aryl chlorides ...

Scheme 29: Photochemical nickel-catalyzed C(sp3)–H methylations.

Scheme 30: Photoredox nickel catalysis-enabled alkylation of unactivated C(sp3)–H bonds with alkyl bromides.

Scheme 31: Photochemical C(sp3)–H alkenylation with alkenyl tosylates.

Scheme 32: Photoredox nickel-catalyzed hydroalkylation of internal alkynes.

Figure 15: Proposed mechanism for the photoredox nickel-catalyzed hydroalkylation of internal alkynes.

Scheme 33: Photoredox nickel-catalyzed hydroalkylation of activated alkynes with C(sp3)−H bonds.

Scheme 34: Allylation of unactivated C(sp3)−H bonds with allylic chlorides.

Scheme 35: Photochemical nickel-catalyzed α-amino C(sp3)–H allylation of secondary amides with trifluoromethyl...

Scheme 36: Photoredox δ C(sp3)‒H allylation of secondary amides with trifluoromethylated alkenes.

Scheme 37: Photoredox nickel-catalyzed acylation of α-amino C(sp3)‒H bonds of N-arylamines.

Figure 16: Proposed mechanism for the photoredox nickel-catalyzed acylation of α-amino C(sp3)–H bonds of N-ary...

Scheme 38: Photocatalytic α‑acylation of ethers with acid chlorides.

Figure 17: Proposed mechanism for the photocatalytic α‑acylation of ethers with acid chlorides.

Scheme 39: Photoredox and nickel-catalyzed C(sp3)‒H esterification with chloroformates.

Scheme 40: Photoredox nickel-catalyzed dehydrogenative coupling of benzylic and aldehydic C–H bonds.

Figure 18: Proposed reaction pathway for the photoredox nickel-catalyzed dehydrogenative coupling of benzylic ...

Scheme 41: Photoredox nickel-catalyzed enantioselective acylation of α-amino C(sp3)–H bonds with carboxylic ac...

Scheme 42: Nickel-catalyzed C(sp3)‒H acylation with N-acylsuccinimides.

Figure 19: Proposed mechanism for the nickel-catalyzed C(sp3)–H acylation with N-acylsuccinimides.

Scheme 43: Nickel-catalyzed benzylic C–H functionalization with acid chlorides 45.

Scheme 44: Photoredox nickel-catalyzed benzylic C–H acylation with N-acylsuccinimides 84.

Scheme 45: Photoredox nickel-catalyzed acylation of indoles 86 with α-oxoacids 87.

Scheme 46: Nickel-catalyzed aldehyde C–H functionalization.

Figure 20: Proposed catalytic cycle for the photoredox nickel-catalyzed aldehyde C–H functionalization.

Scheme 47: Photoredox carboxylation of methylbenzenes with CO2.

Figure 21: Proposed mechanism for the photoredox carboxylation of methylbenzenes with CO2.

Scheme 48: Decatungstate photo-HAT and nickel catalysis enabled alkene difunctionalization.

Figure 22: Proposed catalytic cycle for the decatungstate photo-HAT and nickel catalysis enabled alkene difunc...

Scheme 49: Diaryl ketone HAT catalysis and nickel catalysis enabled dicarbofunctionalization of alkenes.

Figure 23: Proposed catalytic mechanism for the diaryl ketone HAT catalysis and nickel catalysis enabled dicar...

Scheme 50: Overview of photoredox nickel-catalyzed C–H functionalizations.
Icilio Guareschi and his amazing “1897 reaction”
- Gian Cesare Tron,
- Alberto Minassi,
- Giovanni Sorba,
- Mara Fausone and
- Giovanni Appendino
Beilstein J. Org. Chem. 2021, 17, 1335–1351, doi:10.3762/bjoc.17.93
- reactive intermediate capable of transferring a phosphate group to ATP. The Guareschi “1897 reaction” shows a basically different strategy to generate reductive power, associated to aromatization of a prearomatic substrate. The chemiosmotic generation of energy is as universal as the genetic code, and the

Graphical Abstract

Figure 1: Icilio Guareschi (1847–1918). (Source: Annali della Reale Accademia di Agricoltura di Torino 1919, ...

Scheme 1: Vitamin B6 (pyridoxine, 1), gabapentin (2), and thymol (3).

Figure 2: Baliatico (Nursing) by Francesco Scaramuzza (275 cm × 214 cm, Parma, Complesso Museale della Pilott...

Figure 3: Schiff’s fictitious report on the foundation of the Gazzetta Chimica Italiana (Image reproduced fro...

Scheme 2: Reaction of thymol (3) with chloroform under the basic conditions of the Guareschi–Lustgarten react...

Figure 4: The chemistry building of Turin University in a historical picture. Note, that one of the “mysterio...

Scheme 3: Triacetonamine (6) and the related compounds phorone (7), α-eucaine (8), and tropinone (9).

Scheme 4: Taxonomy of the Guareschi pyridone syntheses.

Scheme 5: The catalytic cycle of the “1897 reaction”.

Scheme 6: Resonance forms of the radical 10.

Figure 5: The wet chamber used by Guareschi to restore parchments (Gorrini, G. L'incendio della R. Biblioteca...

Figure 6: The Guareschi mask. (Servizio Chimico Militare. L'opera di Icilio Guareschi precursore della masche...

Figure 7: Guareschi’s bust at the Dipartimento di Scienza e Tecnologia del Farmaco of Turin University. Permi...
CF3-substituted carbocations: underexploited intermediates with great potential in modern synthetic chemistry
- Anthony J. Fernandes,
- Armen Panossian,
- Bastien Michelet,
- Agnès Martin-Mingot,
- Frédéric R. Leroux and
- Sébastien Thibaudeau
Beilstein J. Org. Chem. 2021, 17, 343–378, doi:10.3762/bjoc.17.32


Graphical Abstract

Figure 1: Stabilizing interaction in the CF3CH2+ carbenium ion (top) and structure of the first observable fl...

Scheme 1: Isodesmic equations accounting for the destabilizing effect of the CF3 group. ΔE in kcal⋅mol−1, cal...

Scheme 2: Stabilizing effect of fluorine atoms by resonance electron donation in carbenium ions (δ in ppm).

Scheme 3: Direct in situ NMR observation of α-(trifluoromethyl)carbenium ion or protonated alcohols. Δδ = δ19...

Scheme 4: Reported 13C NMR chemical shifts for the α-(trifluoromethyl)carbenium ion 10c (δ in ppm).

Scheme 5: Direct NMR observation of α-(trifluoromethyl)carbenium ions in situ (δ in ppm).

Scheme 6: Illustration of the ion pair solvolysis mechanism for sulfonate 13f. YOH = solvent.

Figure 2: Solvolysis rate for 13a–i and 17.

Figure 3: Structures of allyl triflates 18 and 19 and allyl brosylate 20. Bs = p-BrC6H4SO2.

Figure 4: Structure of tosylate derivatives 21.

Figure 5: a) Structure of triflate derivatives 22. b) Stereochemistry outcomes of the reaction starting from (...

Scheme 7: Solvolysis reaction of naphthalene and anthracenyl derivatives 26 and 29.

Figure 6: Structure of bisarylated derivatives 34.

Figure 7: Structure of bisarylated derivatives 36.

Scheme 8: Reactivity of 9c in the presence of a Brønsted acid.

Scheme 9: Cationic electrocyclization of 38a–c under strongly acidic conditions.

Scheme 10: Brønsted acid-catalyzed synthesis of indenes 42 and indanes 43.

Scheme 11: Reactivity of sulfurane 44 in triflic acid.

Scheme 12: Solvolysis of triflate 45f in alcoholic solvents.

Scheme 13: Synthesis of labeled 18O-52.

Scheme 14: Reactivity of sulfurane 53 in triflic acid.

Figure 8: Structure of tosylates 56 and 21f.

Scheme 15: Resonance forms in benzylic carbenium ions.

Figure 9: Structure of pyrrole derivatives 58 and 59.

Scheme 16: Resonance structure 60↔60’.

Scheme 17: Ga(OTf)3-catalyzed synthesis of 3,3’- and 3,6’-bis(indolyl)methane from trifluoromethylated 3-indol...

Scheme 18: Proposed reaction mechanism.

Scheme 19: Metal-free 1,2-phosphorylation of 3-indolylmethanols.

Scheme 20: Superacid-mediated arylation of thiophene derivatives.

Scheme 21: In situ mechanistic NMR investigations.

Scheme 22: Proposed mechanisms for the prenyltransferase-catalyzed condensation.

Scheme 23: Influence of a CF3 group on the allylic SN1- and SN2-mechanism-based reactions.

Scheme 24: Influence of the CF3 group on the condensation reaction.

Scheme 25: Solvolysis of 90 in TFE.

Scheme 26: Solvolysis of allyl triflates 94 and 97 and isomerization attempt of 96.

Scheme 27: Proposed mechanism for the formation of 95.

Scheme 28: Formation of α-(trifluoromethyl)allylcarbenium ion 100 in a superacid.

Scheme 29: Lewis acid activation of CF3-substituted allylic alcohols.

Scheme 30: Bimetallic-cluster-stabilized α-(trifluoromethyl)carbenium ions.

Scheme 31: Reactivity of cluster-stabilized α-(trifluoromethyl)carbenium ions.

Scheme 32: α-(Trifluoromethyl)propargylium ion 122↔122’ generated from silyl ether 120 in a superacid.

Scheme 33: Formation of α-(trifluoromethyl)propargylium ions from CF3-substituted propargyl alcohols.

Scheme 34: Direct NMR observation of the protonation of some trifluoromethyl ketones in situ and the correspon...

Scheme 35: Selected resonance forms in protonated fluoroketone derivatives.

Scheme 36: Acid-catalyzed Friedel–Crafts reactions of trifluoromethyl ketones 143a,b and 147a–c.

Scheme 37: Enantioselective hydroarylation of CF3-substituted ketones.

Scheme 38: Acid-catalyzed arylation of ketones 152a–c.

Scheme 39: Reactivity of 156 in a superacid.

Scheme 40: Reactivity of α-CF3-substituted heteroaromatic ketones and alcohols as well as 1,3-diketones.

Scheme 41: Reactivity of 168 with benzene in the presence of a Lewis or Brønsted acid.

Scheme 42: Acid-catalyzed three-component asymmetric reaction.

Scheme 43: Anodic oxidation of amines 178a–c and proposed mechanism.

Scheme 44: Reactivity of 179b in the presence of a strong Lewis acid.

Scheme 45: Trifluoromethylated derivatives as precursors of trifluoromethylated iminium ions.

Scheme 46: Mannich reaction with trifluoromethylated hemiaminal 189.

Scheme 47: Suitable nucleophiles reacting with 192 after Lewis acid activation.

Scheme 48: Strecker reaction involving the trifluoromethylated iminium ion 187.

Scheme 49: Reactivity of 199 toward nucleophiles.

Scheme 50: Reactivity of 204a with benzene in the presence of a Lewis acid.

Scheme 51: Reactivity of α-(trifluoromethyl)-α-chloro sulfides in the presence of strong Lewis acids.

Scheme 52: Anodic oxidation of sulfides 213a–h and Pummerer rearrangement.

Scheme 53: Mechanism for the electrochemical oxidation of the sulfide 213a.

Scheme 54: Reactivity of (trifluoromethyl)diazomethane (217a) in HSO3F.

Figure 10: a) Structure of diazoalkanes 217a–c and b) rate-limiting steps of their decomposition.

Scheme 55: Deamination reaction of racemic 221 and enantioenriched (S)-221.

Scheme 56: Deamination reaction of labeled 221-d2. Elimination products were formed in this reaction, the yiel...

Scheme 57: Deamination reaction of 225-d2. Elimination products were also formed in this reaction in undetermi...

Scheme 58: Formation of 229 from 228 via 1,2-H-shift.

Scheme 59: Deamination reaction of 230. Elimination products were formed in this reaction, the yield of which ...

Scheme 60: Deamination of several diazonium ions. Elimination products were formed in these reactions, the yie...

Scheme 61: Solvolysis reaction mechanism of alkyl tosylates.

Scheme 62: Solvolysis outcome for the tosylates 248 and 249 in HSO3FSbF5.

Figure 11: Solvolysis rate of 248, 249, 252, and 253 in 91% H2SO4.

Scheme 63: Illustration of the reaction pathways. TsCl, pyridine, −5 °C (A); 98% H2SO4, 30 °C (B); 98% H2SO4, ...

Scheme 64: Proposed solvolysis mechanism for the aliphatic tosylate 248.

Scheme 65: Solvolysis of the derivatives 259 and 260.

Scheme 66: Solvolysis of triflate 261. SOH = solvent.

Scheme 67: Intramolecular Friedel–Crafts alkylations upon the solvolysis of triflates 264 and 267.

Scheme 68: α-CF3-enhanced γ-silyl elimination of cyclobutyltosylates 270a,b.

Scheme 69: γ-Silyl elimination in the synthesis of a large variety of CF3-substituted cyclopropanes. Pf = pent...

Scheme 70: Synthetic pathways to 281. aNMR yields.

Scheme 71: The cyclopropyl-substituted homoallylcyclobutylcarbenium ion manifold.

Scheme 72: Reactivity of CF3-substituted cyclopropylcarbinyl derivatives 287a–c. LG = leaving group.

Scheme 73: Reactivity of CF3-substituted cyclopropylcarbinyl derivatives 291a–c.

Scheme 74: Superacid-promoted dimerization or TFP.

Scheme 75: Reactivity of TFP in a superacid.

Scheme 76: gem-Difluorination of α-fluoroalkyl styrenes via the formation of a “hidden” α-RF-substituted carbe...

Scheme 77: Solvolysis of CF3-substituted pentyne 307.

Scheme 78: Photochemical rearrangement of 313.

Figure 12: Structure of 2-norbornylcarbenium ion 318 and argued model for the stabilization of this cation.

Figure 13: Structures and solvolysis rate (TFE, 25 °C) of the sulfonates 319–321. Mos = p-MeOC6H4SO2.

Scheme 79: Mechanism for the solvolysis of 323. SOH = solvent.

Scheme 80: Products formed by the hydrolysis of 328.

Scheme 81: Proposed carbenium ion intermediates in an equilibrium during the solvolysis of tosylates 328, 333,...
The preparation and properties of 1,1-difluorocyclopropane derivatives
- Kymbat S. Adekenova,
- Peter B. Wyatt and
- Sergazy M. Adekenov
Beilstein J. Org. Chem. 2021, 17, 245–272, doi:10.3762/bjoc.17.25

Graphical Abstract

Scheme 1: Synthesis of 1,1-difluoro-2,3-dimethylcyclopropane (2).

Scheme 2: Cyclopropanation via dehydrohalogenation of chlorodifluoromethane.

Scheme 3: Difluorocyclopropanation of methylstyrene 7 using dibromodifluoromethane and zinc.

Scheme 4: Synthesis of difluorocyclopropanes from the reaction of dibromodifluoromethane and triphenylphosphi...

Scheme 5: Generation of difluorocarbene in a catalytic two-phase system and its addition to tetramethylethyle...

Scheme 6: The reaction of methylstyrene 7 with chlorodifluoromethane (11) in the presence of a tetraarylarson...

Scheme 7: Pyrolysis of sodium chlorodifluoroacetate (12) in refluxing diglyme in the presence of alkene 13.

Scheme 8: Synthesis of boron-substituted gem-difluorocyclopropanes 16.

Scheme 9: Addition of sodium bromodifluoroacetate (17) to alkenes.

Scheme 10: Addition of sodium bromodifluoroacetate (17) to silyloxy-substituted cyclopropanes 20.

Scheme 11: Synthesis of difluorinated nucleosides.

Scheme 12: Addition of butyl acrylate (26) to difluorocarbene generated from TFDA (25).

Scheme 13: Addition of difluorocarbene to propargyl esters 27 and conversion of the difluorocyclopropenes 28 t...

Scheme 14: The generation of difluorocyclopropanes using MDFA 30.

Scheme 15: gem-Difluorocyclopropanation of styrene (32) using difluorocarbene generated from TMSCF3 (31) under...

Scheme 16: Synthesis of a gem-difluorocyclopropane derivative using HFPO (41) as a source of difluorocarbene.

Scheme 17: Cyclopropanation of (Z)-2-butene in the presence of difluorodiazirine (44).

Scheme 18: The cyclopropanation of 1-octene (46) using Seyferth's reagent (45) as a source of difluorocarbene.

Scheme 19: Alternative approaches for the difluorocarbene synthesis from trimethyl(trifluoromethyl)tin (48).

Scheme 20: Difluorocyclopropanation of cyclohexene (49).

Scheme 21: Synthesis of difluorocyclopropane derivative 53 using bis(trifluoromethyl)cadmium (51) as the diflu...

Scheme 22: Addition of difluorocarbene generated from tris(trifluoromethyl)bismuth (54).

Scheme 23: Addition of a stable (trifluoromethyl)zinc reagent to styrenes.

Scheme 24: The preparation of 2,2-difluorocyclopropanecarboxylic acids of type 58.

Scheme 25: Difluorocyclopropanation via Michael cyclization.

Scheme 26: Difluorocyclopropanation using N-acylimidazolidinone 60.

Scheme 27: Difluorocyclopropanation through the cyclization of phenylacetonitrile (61) and 1,2-dibromo-1,1-dif...

Scheme 28: gem-Difluoroolefins 64 for the synthesis of functionalized cyclopropanes 65.

Scheme 29: Preparation of aminocyclopropanes 70.

Scheme 30: Synthesis of fluorinated methylenecyclopropane 74 via selenoxide elimination.

Scheme 31: Reductive dehalogenation of (1R,3R)-75.

Scheme 32: Synthesis of chiral monoacetates by lipase catalysis.

Scheme 33: Transformation of (±)-trans-81 using Rhodococcus sp. AJ270.

Scheme 34: Transformation of (±)-trans-83 using Rhodococcus sp. AJ270.

Scheme 35: Hydrogenation of difluorocyclopropenes through enantioselective hydrocupration.

Scheme 36: Enantioselective transfer hydrogenation of difluorocyclopropenes with a Ru-based catalyst.

Scheme 37: The thermal transformation of trans-1,2-dichloro-3,3-difluorocyclopropane (84).

Scheme 38: cis–trans-Epimerization of 1,1-difluoro-2,3-dimethylcyclopropane.

Scheme 39: 2,2-Difluorotrimethylene diradical intermediate.

Scheme 40: Ring opening of stereoisomers 88 and 89.

Scheme 41: [1,3]-Rearrangement of alkenylcyclopropanes 90–92.

Scheme 42: Thermolytic rearrangement of 2,2-difluoro-1-vinylcyclopropane (90).

Scheme 43: Thermal rearrangement for ethyl 3-(2,2-difluoro)-3-phenylcyclopropyl)acrylates 93 and 95.

Scheme 44: Possible pathways of the ring opening of 1,1-difluoro-2-vinylcyclopropane.

Scheme 45: Equilibrium between 1,1-difluoro-2-methylenecyclopropane (96) and (difluoromethylene)cyclopropane 97...

Scheme 46: Ring opening of substituted 1,1-difluoro-2,2-dimethyl-3-methylenecyclopropane 98.

Scheme 47: 1,1-Difluorospiropentane rearrangement.

Scheme 48: Acetolysis of (2,2-difluorocyclopropyl)methyl tosylate (104) and (1,1-difluoro-2-methylcyclopropyl)...

Scheme 49: Ring opening of gem-difluorocyclopropyl ketones 106 and 108 by thiolate nucleophiles.

Scheme 50: Hydrolysis of gem-difluorocyclopropyl acetals 110.

Scheme 51: Ring-opening reaction of 2,2-difluorocyclopropyl ketones 113 in the presence of ionic liquid as a s...

Scheme 52: Ring opening of gem-difluorocyclopropyl ketones 113a by MgI2-initiated reaction with diarylimines 1...

Scheme 53: Ring-opening reaction of gem-difluorocyclopropylstannanes 117.

Scheme 54: Preparation of 1-fluorovinyl vinyl ketone 123 and the synthesis of 2-fluorocyclopentenone 124. TBAT...

Scheme 55: Iodine atom-transfer ring opening of 1,1-difluoro-2-(1-iodoalkyl)cyclopropanes 125a–c.

Scheme 56: Ring opening of bromomethyl gem-difluorocyclopropanes 130 and formation of gem-difluoromethylene-co...

Scheme 57: Ring-opening aerobic oxidation reaction of gem-difluorocyclopropanes 132.

Scheme 58: Dibrominative ring-opening functionalization of gem-difluorocyclopropanes 134.

Scheme 59: The selective formation of (E,E)- and (E,Z)-fluorodienals 136 and 137 from difluorocyclopropyl acet...

Scheme 60: Proposed mechanism for the reaction of difluoro(methylene)cyclopropane 139 with Br2.

Scheme 61: Thermal rearrangement of F2MCP 139 and iodine by CuI catalysis.

Scheme 62: Synthesis of 2-fluoropyrroles 142.

Scheme 63: Ring opening of gem-difluorocyclopropyl ketones 143 mediated by BX3.

Scheme 64: Lewis acid-promoted ring-opening reaction of 2,2-difluorocyclopropanecarbonyl chloride (148).

Scheme 65: Ring-opening reaction of the gem-difluorocyclopropyl ketone 106 by methanolic KOH.

Scheme 66: Hydrogenolysis of 1,1-difluoro-3-methyl-2-phenylcyclopropane (151).

Scheme 67: Synthesis of monofluoroalkenes 157.

Scheme 68: The stereoselective Ag-catalyzed defluorinative ring-opening diarylation of 1-trimethylsiloxy-2,2-d...

Scheme 69: Synthesis of 2-fluorinated allylic compounds 162.

Scheme 70: Pd-catalyzed cross-coupling reactions of gem-difluorinated cyclopropanes 161.

Scheme 71: The (Z)-selective Pd-catalyzed ring-opening sulfonylation of 2-(2,2-difluorocyclopropyl)naphthalene...

Figure 1: Structures of zosuquidar hydrochloride and PF-06700841.

Scheme 72: Synthesis of methylene-gem-difluorocyclopropane analogs of nucleosides.

Figure 2: Anthracene-difluorocyclopropane hybrid derivatives.

Figure 3: Further examples of difluorcyclopropanes in modern drug discovery.
Azo-dimethylaminopyridine-functionalized Ni(II)-porphyrin as a photoswitchable nucleophilic catalyst
- Jannis Ludwig,
- Julian Helberg,
- Hendrik Zipse and
- Rainer Herges
Beilstein J. Org. Chem. 2020, 16, 2119–2126, doi:10.3762/bjoc.16.179

- -porphyrin. The DMAP-catalyzed acetylation of alcohols should not suffer from this drawback because the reactive intermediate (acylpyridinium cation) is positively charged and does not coordinate to the Ni2+ ion [33]. Another promising approach towards coordination-based, photoswitchable catalysts are

Graphical Abstract

Figure 1: Basicity and nucleophilicity switching of the 4-(N,N-dimethylamino)pyridine “record player” molecul...

Scheme 1: Synthesis of 4-N,N-dimethylamino record player molecule 1 by Suzuki reaction between Ni-porphyrin p...

Figure 2: Composition of the different states of porphyrin 1 (1 mM) in the PSS at 530 nm and 435 nm, determin...

Figure 3: a) UV–vis cuvette with a solution of porphyrin 1 (13.1 µM in THF) and the corresponding UV–vis spec...

Scheme 2: General scheme of the nitroaldol (Henry) reaction that was used to investigate photoswitchable cata...

Scheme 3: DMAP (2), azopyridine trans-4, record player trans- and cis-1 and Ni-porphyrin 8 were used in kinet...

Figure 4: Conversion of 4-nitrobenzaldehyde (6) in the Henry reaction with nitroethane (5) as a function of t...
[3 + 2] Cycloaddition with photogenerated azomethine ylides in β-cyclodextrin
- Margareta Sohora,
- Leo Mandić and
- Nikola Basarić
Beilstein J. Org. Chem. 2020, 16, 1296–1304, doi:10.3762/bjoc.16.110
- . Irradiation of such a complex leads to decarboxylation and formation of the reactive intermediate, azomethine ylide, within the supramolecular host. The subsequent [3 + 2] cycloaddition within the inclusion complex gives heterocyclic cycloadducts, even though it is conducted in aqueous solvent in which ylides

Graphical Abstract

Figure 1: Phthalimide derivatives 1–3 and the corresponding azomethine ylides 1AMY-3AMY.

Scheme 1: Irradiation of 1 in the presence of acrylonitrile (AN).

Figure 2: Dependence of the chemical shift of the H-atom at the cyclohexane 2 position in compound 2 on the β...

Scheme 2: Complexation of 2 with β-CD, and formation of a ternary complex AN@2@β-CD.

Scheme 3: Photochemistry of 2 in the presence of AN, with or without β-CD.

Scheme 4: Photochemistry of 3 in the presence of AN, with or without β-CD.
Photocatalysis with organic dyes: facile access to reactive intermediates for synthesis
- Stephanie G. E. Amos,
- Marion Garreau,
- Luca Buzzetti and
- Jerome Waser
Beilstein J. Org. Chem. 2020, 16, 1163–1187, doi:10.3762/bjoc.16.103
- each of these steps, the role of A or D is assumed by a redox-active agent, either the substrate, a sacrificial electron donor/acceptor, or a reactive intermediate. This approach, usually named photoredox catalysis, has known a remarkable growth in the last decade and has given access to both neutral
- -systems: heteroarenes and electron-poor alkenes in particular. Using the tools of organophotocatalysis, this reactive intermediate can be directly obtained from carbonyl derivatives, such as aldehydes, α-keto acids, oxalates, or carbamates through oxidative or reductive decarboxylations, oxidative

Graphical Abstract

Figure 1: Selected examples of organic dyes. Mes-Acr+: 9-mesityl-10-methylacridinium, DCA: 9,10-dicyanoanthra...

Scheme 1: Activation modes in photocatalysis.

Scheme 2: Main strategies for the formation of C(sp3) radicals used in organophotocatalysis.

Scheme 3: Illustrative example for the photocatalytic oxidative generation of radicals from carboxylic acids:...

Scheme 4: Illustrative example for the photocatalytic reductive generation of C(sp3) radicals from redoxactiv...

Figure 2: Common substrates for the photocatalytic oxidative generation of C(sp3) radicals.

Scheme 5: Illustrative example for the photocatalytic oxidative generation of radicals from dihydropyridines ...

Scheme 6: Illustrative example for the photocatalytic oxidative generation of C(sp3) radicals from trifluorob...

Scheme 7: Illustrative example for the photocatalytic reductive generation of C(sp3) radicals from benzylic h...

Scheme 8: Illustrative example for the photocatalytic generation of C(sp3) radicals via direct HAT: the cross...

Scheme 9: Illustrative example for the photocatalytic generation of C(sp3) radicals via indirect HAT: the deu...

Scheme 10: Selected precursors for the generation of aryl radicals using organophotocatalysis.

Scheme 11: Illustrative example for the photocatalytic reductive generation of aryl radicals from aryl diazoni...

Scheme 12: Illustrative examples for the photocatalytic reductive generation of aryl radicals from haloarenes:...

Scheme 13: Illustrative example for the photocatalytic reductive generation of aryl radicals from aryl halides...

Scheme 14: Illustrative example for the photocatalytic reductive generation of aryl radicals from arylsulfonyl...

Scheme 15: Illustrative example for the reductive photocatalytic generation of aryl radicals from triaryl sulf...

Scheme 16: Main strategies towards acyl radicals used in organophotocatalysis.

Scheme 17: Illustrative example for the decarboxylative photocatalytic generation of acyl radicals from α-keto...

Scheme 18: Illustrative example for the oxidative photocatalytic generation of acyl radicals from acyl silanes...

Scheme 19: Illustrative example for the oxidative photocatalytic generation of carbamoyl radicals from 4-carba...

Scheme 20: Illustrative example of the photocatalytic HAT approach for the generation of acyl radicals from al...

Scheme 21: General reactivity of a) radical cations; b) radical anions; c) the main strategies towards aryl an...

Scheme 22: Illustrative example for the oxidative photocatalytic generation of alkene radical cations from alk...

Scheme 23: Illustrative example for the reductive photocatalytic generation of an alkene radical anion from al...

Figure 3: Structure of C–X radical anions and their neutral derivatives.

Scheme 24: Illustrative example for the photocatalytic reduction of imines and the generation of an α-amino C(...

Scheme 25: Illustrative example for the oxidative photocatalytic generation of aryl radical cations from arene...

Scheme 26: NCR classifications and generation.

Scheme 27: Illustrative example for the photocatalytic reductive generation of iminyl radicals from O-aryl oxi...

Scheme 28: Illustrative example for the photocatalytic oxidative generation of iminyl radicals from α-N-oxy ac...

Scheme 29: Illustrative example for the photocatalytic oxidative generation of iminyl radicals via an N–H bond...

Scheme 30: Illustrative example for the photocatalytic oxidative generation of amidyl radicals from Weinreb am...

Scheme 31: Illustrative example for the photocatalytic reductive generation of amidyl radicals from hydroxylam...

Scheme 32: Illustrative example for the photocatalytic reductive generation of amidyl radicals from N-aminopyr...

Scheme 33: Illustrative example for the photocatalytic oxidative generation of amidyl radicals from α-amido-ox...

Scheme 34: Illustrative example for the photocatalytic oxidative generation of aminium radicals: the N-aryltet...

Scheme 35: Illustrative example for the photocatalytic oxidative generation of nitrogen-centered radical catio...

Scheme 36: Illustrative example for the photocatalytic oxidative generation of nitrogen-centered radical catio...

Scheme 37: Illustrative example for the photocatalytic oxidative generation of hydrazonyl radical from hydrazo...

Scheme 38: Generation of O-radicals.

Scheme 39: Illustrative examples for the photocatalytic generation of O-radicals from N-alkoxypyridinium salts...

Scheme 40: Illustrative examples for the photocatalytic generation of O-radicals from alkyl hydroperoxides: th...

Scheme 41: Illustrative example for the oxidative photocatalytic generation of thiyl radicals from thiols: the...

Scheme 42: Main strategies and reagents for the generation of sulfonyl radicals used in organophotocatalysis.

Scheme 43: Illustrative example for the reductive photocatalytic generation of sulfonyl radicals from arylsulf...

Scheme 44: Illustrative example of a Cl atom abstraction strategy for the photocatalytic generation of sulfamo...

Scheme 45: Illustrative example for the oxidative photocatalytic generation of sulfonyl radicals from sulfinic...

Scheme 46: Illustrative example for the photocatalytic generation of electronically excited triplet states: th...

Scheme 47: Illustrative example for the photocatalytic generation of electronically excited triplet states: th...
Cation-induced ring-opening and oxidation reaction of photoreluctant spirooxazine–quinolizinium conjugates
- Phil M. Pithan,
- Sören Steup and
- Heiko Ihmels
Beilstein J. Org. Chem. 2020, 16, 904–916, doi:10.3762/bjoc.16.82

- initial development of a broad absorption band between 500 nm and 650 nm was also observed, which again gradually disappeared during the course of the reaction. These observations pointed towards the formation of the red-colored, open merocyanine form as a very reactive intermediate during the reaction

Graphical Abstract

Scheme 1: Photo- or cation-induced ring-opening reaction of spirooxazine 1aSO; Mn+ = Pb2+, La3+, Eu3+, Tb3+ [17].

Scheme 2: Synthesis of the spirooxazine–quinolizinium conjugates 3a and 3b.

Figure 1: Colors of the solutions resulting from the addition of metal ions (c = 50 µM) to derivative 3a (c =...

Figure 2: Spectrophotometric titration of 3a (A) and 3b (B) (c = 20 µM) with Cu(BF4)2 (c = 2.44 mM) in MeCN. ...

Figure 3: Absorption (A) and fluorescence spectrum (B) of 3a in MeCN (c = 5 µM) in the absence (black) and in...

Figure 4: Emission colors of solutions resulting from the addition of metal ions (c = 50 µM) to derivative 3a...

Scheme 3: Cu2+-induced formation of the oxazole derivatives 4a and 4b.

Figure 5: 1H NMR spectra (600 MHz, 6.4–9.4 ppm) of 3a (c = 2.0 mM) in the absence (A) and in the presence (B–...

Figure 6: Spectral changes of 3a (c = 20 µM) upon the addition of Cu2+ (A) and Fe3+ (B) (cM+ = 60 µM) in MeCN...

Scheme 4: Proposed mechanism for the formation of the oxazole derivatives 4a and 4b (cf. Scheme 3); Mn+ = Cu2+, Fe3+,...
The use of isoxazoline and isoxazole scaffolding in the design of novel thiourea and amide liquid-crystalline compounds
- Itamar L. Gonçalves,
- Rafaela R. da Rosa,
- Vera L. Eifler-Lima and
- Aloir A. Merlo
Beilstein J. Org. Chem. 2020, 16, 175–184, doi:10.3762/bjoc.16.20
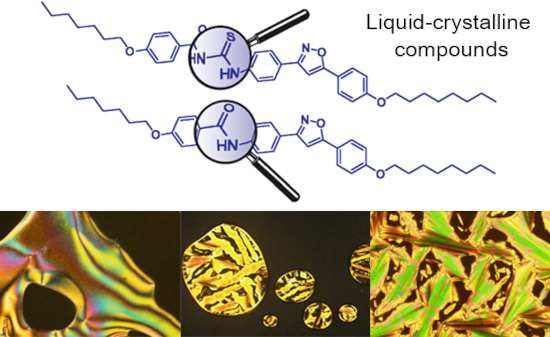
- ]+ during the condensation reaction of thiocyanate salt, a reaction test was performed to confirm the reactivity between the amines and acyl chloride. This involved the formation and isolation of amide 24, as a result of the reaction between the reactive intermediate nonyloxybenzoyl chloride from acid 23

Graphical Abstract

Scheme 1: Amines 3, 4, 8, 9, 12 and 13 installed on 5-membered isoxazoline and isoxazole rings.

Scheme 2: Synthesis of acylisoxazolinylthioureas 17a–c and acylisoxazolylthioureas 18a–c. (i) SOCl2, reflux, ...

Scheme 3: Synthesis of amides. Part A: (i) SOCl2, reflux; (ii) KOCN, acetone, reflux; (iii) amines 3, 4, 8 an...

Figure 1: Optical textures observed on POM for thioureas 17a (a), 17b (b), 18a (c), 18b (d) and 18c (e,f). Al...

Figure 2: Optical textures observed on POM of amide 19. (a) Fan-shaped focal conic texture of the SmA mesopha...

Figure 3: DSC curves for the thiourea 17a (A), amide 19 (B) and 20 (C) upon the first heating and cooling cur...

Figure 4: TGA analysis for thiourea 18c; and amides 20 and 22.
Selectivity in multiple multicomponent reactions: types and synthetic applications
- Ouldouz Ghashghaei,
- Francesca Seghetti and
- Rodolfo Lavilla
Beilstein J. Org. Chem. 2019, 15, 521–534, doi:10.3762/bjoc.15.46
- step in the process leads to a less reactive intermediate, the system may stop temporarily at this level, and once completed, a second MCR with a distinct set of reagents may take place under more vigorous activation. This allows for selective sequential MMCRs, although there is a clear dependency on

Graphical Abstract

Scheme 1: Selectivity levels found in multiple multicomponent reactions. I) Innate selectivity; II) sequentia...

Scheme 2: Indiscriminate double Ugi MCR upon pyridine-2,6-dicarboxylic acid.

Scheme 3: Representative examples of MCR-polymer synthesis. A) Biginelli HTS of polymers; B) Passerini;- C) U...

Scheme 4: Concept of multicomponent macrocyclization.

Scheme 5: Supramolecular structures out of MMCR macrocyclizations.

Scheme 6: Macrocyclization by MMCRs. A) Staudinger MCR; B) boronic-imine MCR.

Scheme 7: Selective Sequential MMCRs. A and B) MCRs involving terephthalaldehyde; C) Multiple GBB processes w...

Scheme 8: Biased substrates for selective MMCRs.

Scheme 9: The Union concept. A) Asinger–Ugi combination; B) Passerini–Ugi/azide from anthranilic acid; C) Pas...

Scheme 10: Relevant examples of consecutive MCRs exploiting the Union Concept. A) Petasis-Ugi combination; B) ...

Scheme 11: Selective MMCRs featuring FGs with distinct reactivity along the sequence. A) Synthesis of aminomet...

Scheme 12: High order MMCRs. A) Ugi/Ugi–Smiles 7C combination; B) imidazoline-N-cyanomethylamide-Ugi union lea...

Scheme 13: Consecutive Ugi 4CR-deprotection–Ugi 4CR strategy towards A) PNA oligomers and B) peptidic tetrazol...

Scheme 14: Sequential Ugi 4CR-deprotection access to cyclopeptoids.

Scheme 15: Stepwise access to 6-aminopenicillanic acid derivative through an Asinger, deprotection, Joullié ap...

Scheme 16: A triple MCR-deprotection approach affording anticancer peptidomimetics.
Study on the regioselectivity of the N-ethylation reaction of N-benzyl-4-oxo-1,4-dihydroquinoline-3-carboxamide
- Pedro N. Batalha,
- Luana da S. M. Forezi,
- Maria Clara R. Freitas,
- Nathalia M. de C. Tolentino,
- Ednilsom Orestes,
- José Walkimar de M. Carneiro,
- Fernanda da C. S. Boechat and
- Maria Cecília B. V. de Souza
Beilstein J. Org. Chem. 2019, 15, 388–400, doi:10.3762/bjoc.15.35

- the oxoquinoline, when compared to that of the carboxamide group. Because of this, the treatment with the base produces the reactive intermediate, the conjugate base resulting from the deprotonation of the oxoquinoline core, probably exclusively, which acts as the nucleophile in the SN2 reaction with

Graphical Abstract

Figure 1: Structures of some bioactive 4-oxoquinoline-3-carboxamide derivatives 1–4 with different bioactive ...

Figure 2: Structural modifications on the 4-oxo-1,4-dihydroquinoline-3-carboxamide scaffold.

Scheme 1: Synthetic route for the preparation of 1-ethyl-4-oxoquinoline-3-carboxamide 7.

Scheme 2: Reaction steps and main transition state leading to compound 7.

Figure 3: Same scale partial 1H NMR spectra of compounds 5 and 7 (DMSO-d6, 500 MHz).

Figure 4: 1H,1H-COSY spectrum of derivative 7 (DMSO-d6, 500 MHz).

Figure 5: Partial HMBC spectrum of derivative 7 (DMSO-d6, 500 MHz).

Figure 6: Asymmetric unit of product 7.

Scheme 3: Deprotonation of 5 forming 8a and 8b, followed by reaction with bromoethane leading to products 7 a...

Scheme 4: Acid–base equilibria considered for the data displayed in Table 2.

Scheme 5: Charge dispersion due to resonance effects for both deprotonated species.
Annulation of 1H-pyrrole-2,3-diones by thioacetamide: an approach to 5-azaisatins
- Aleksandr I. Kobelev,
- Ekaterina E. Stepanova,
- Maksim V. Dmitriev and
- Andrey N. Maslivets
Beilstein J. Org. Chem. 2019, 15, 364–370, doi:10.3762/bjoc.15.32
- chromatography. Moreover, the resulting compounds may be of pharmaceutical interest and can be starting materials for the synthesis of various aza analogs of isatin-based heterocycles. It also should be mentioned that we have observed an interesting thioacetamide-based reactive intermediate that can possibly

Graphical Abstract

Scheme 1: Approaches to the synthesis of the 5-azaisatin core.

Scheme 2: Our previous work on the interaction of PBTs 2 with thioamides.

Scheme 3: Interaction of PBTs 2 with thioacetamide.

Scheme 4: Plausible pathways for the formation of compound 4.

Scheme 5: Experiments on the intermolecular trapping of spiro[thiazolo-5,2'-pyrrole] 3a.

Scheme 6: Exploration of the substrate scope.

Scheme 7: Interaction of PBT 2a with N-phenylthioacetamide.
Sigmatropic rearrangements of cyclopropenylcarbinol derivatives. Access to diversely substituted alkylidenecyclopropanes
- Guillaume Ernouf,
- Jean-Louis Brayer,
- Christophe Meyer and
- Janine Cossy
Beilstein J. Org. Chem. 2019, 15, 333–350, doi:10.3762/bjoc.15.29
- rearrangement of cyanate 27 into isocyanate 28 (Scheme 15) [53]. All attempts to isolate isocyanate 28 were unsuccessful but derivatization of this latter reactive intermediate could be achieved in situ by addition of a broad range of nucleophiles, which were either used as co-solvents or added in excess. Thus

Graphical Abstract

Scheme 1: Representative strategies for the formation of alkylidenecyclopropanes from cyclopropenes and scope...

Scheme 2: [2,3]-Sigmatropic rearrangement of phosphinites 2a–h.

Scheme 3: [2,3]-Sigmatropic rearrangement of a phosphinite derived from enantioenriched cyclopropenylcarbinol...

Scheme 4: Selective reduction of phosphine oxide (E)-3f.

Scheme 5: Attempted thermal [2,3]-sigmatropic rearrangement of phosphinite 6a.

Scheme 6: Computed activation barriers and free enthalpies.

Scheme 7: [2,3]-Sigmatropic rearrangement of phosphinites 6a–j.

Scheme 8: Proposed mechanism for the Lewis base-catalyzed rearrangement of phosphinites 6.

Scheme 9: [3,3]-Sigmatropic rearrangement of tertiary cyclopropenylcarbinyl acetates 10a–c.

Scheme 10: [3,3]-Sigmatropic rearrangement of secondary cyclopropenylcarbinyl esters 10d–h.

Scheme 11: [3,3]-Sigmatropic rearrangement of trichoroacetimidates 12a–i.

Scheme 12: Reaction of trichloroacetamide 13f with pyrrolidine.

Scheme 13: Catalytic hydrogenation of (arylmethylene)cyclopropropane 13f.

Scheme 14: Instability of trichloroacetimidates 21a–c derived from cyclopropenylcarbinols 20a–c.

Scheme 15: [3,3]-Sigmatropic rearrangement of cyanate 27 generated from cyclopropenylcarbinyl carbamate 26.

Scheme 16: Synthesis of alkylidene(aminocyclopropane) derivatives 30–37 from carbamate 26.

Scheme 17: Scope of the dehydration–[3,3]-sigmatropic rearrangement sequence of cyclopropenylcarbinyl carbamat...

Scheme 18: Formation of trifluoroacetamide 50 from carbamate 49.

Scheme 19: Formation of alkylidene[(N-trifluoroacetylamino)cyclopropanes] 51–54.

Scheme 20: Diastereoselective hydrogenation of alkylidenecyclopropane 51.

Scheme 21: Ireland–Claisen rearrangement of cyclopropenylcarbinyl glycolates 56a–l.

Scheme 22: Synthesis and Ireland–Claisen rearrangement of glycolate 61 possessing gem-diester substitution at ...

Scheme 23: Synthesis of alkylidene(gem-difluorocyclopropanes) 66a–h, and 66k–n from propargyl glycolates 64a–n....

Scheme 24: Ireland–Claisen rearrangement of N,N-diBoc glycinates 67a and 67b.

Scheme 25: Diastereoselective hydrogenation of alkylidenecyclopropanes 58a and 74.

Scheme 26: Synthesis of functionalized gem-difluorocyclopropanes 76 and 77 from alkylidenecyclopropane 66a.

Scheme 27: Access to oxa- and azabicyclic compounds 78–80.
Cobalt- and rhodium-catalyzed carboxylation using carbon dioxide as the C1 source
- Tetsuaki Fujihara and
- Yasushi Tsuji
Beilstein J. Org. Chem. 2018, 14, 2435–2460, doi:10.3762/bjoc.14.221

- importance, since the highly reactive intermediate can be generated by photochemical reaction such as electron transfer and energy transfer [43][44][45]. Among them, light-energy-driven CO2 fixation reactions via C–C bond formation are promising in terms of mimicking photosynthesis. In 2015, Murakami et al

Graphical Abstract

Scheme 1: Optimization of the Co-catalyzed carboxylation of 1a.

Scheme 2: Co-catalyzed carboxylation of propargyl acetates 1.

Scheme 3: Plausible reaction mechanism for the Co-catalyzed carboxylation of propargyl acetates 1.

Scheme 4: Optimization of the Co-catalyzed carboxylation of 3a.

Scheme 5: Co-catalyzed carboxylation of vinyl triflates 3.

Scheme 6: Co-catalyzed carboxylation of a sterically hindered aryl triflate 5.

Scheme 7: Optimization of the Co-catalyzed carboxylation of 7a.

Scheme 8: Scope of the reductive carboxylation of α,β-unsaturated nitriles 7.

Scheme 9: Scope of the carboxylation of α,β-unsaturated carboxamides 9.

Scheme 10: Optimization of the Co-catalyzed carboxylation of 11a.

Scheme 11: Scope of the carboxylation of allylarenes 11.

Scheme 12: Scope of the carboxylation of 1,4-diene derivatives 14.

Scheme 13: Plausible reaction mechanism for the Co-catalyzed C(sp3)–H carboxylation of allylarenes.

Scheme 14: Optimization of the Co-catalyzed carboxyzincation of 16a.

Scheme 15: Derivatization of the carboxyzincated product.

Scheme 16: Co-catalyzed carboxyzincation of alkynes 16.

Scheme 17: Plausible reaction mechanism for the Co-catalyzed carboxyzincation of alkynes 16.

Scheme 18: Co-catalyzed four-component coupling of alkynes 16, acrylates 18, CO2, and zinc.

Scheme 19: Proposed reaction mechanism for the Co-catalyzed four-component coupling.

Scheme 20: Visible-light-driven hydrocarboxylation of alkynes.

Scheme 21: Visible-light-driven synthesis of γ-hydroxybutenolides from ortho-ester-substituted aryl alkynes.

Scheme 22: One-pot synthesis of coumarines and 2-quinolones via hydrocarboxylation/alkyne isomerization/cycliz...

Scheme 23: Proposed reaction mechanism for the Co-catalyzed carboxylative cyclization of ortho-substituted aro...

Scheme 24: Rh-catalyzed carboxylation of arylboronic esters 25.

Scheme 25: Rh-catalyzed carboxylation of alkenylboronic esters 27.

Scheme 26: Plausible reaction mechanism for the Rh-catalyzed carboxylation of arylboronic esters 25.

Scheme 27: Ligand effect on the Rh-catalyzed carboxylation of 2-phenylpyridine 29a.

Scheme 28: Rh-catalyzed chelation-assisted C(sp2)–H bond carboxylation with CO2.

Scheme 29: Reaction mechanism for the Rh-catalyzed C(sp2)–H carboxylation of 2-pyridylarenes 29.

Scheme 30: Carboxylation of C(sp2)–H bond with CO2.

Scheme 31: Carboxylation of C(sp2)–H bond with CO2.

Scheme 32: Reaction mechanism for the Rh-catalyzed C(sp2)–H carboxylation of 2-arylphenols 34.

Scheme 33: Hydrocarboxylation of styrene derivatives with CO2.

Scheme 34: Hydrocarboxylation of α,β-unsaturated esters with CO2.

Scheme 35: Asymmetric hydrocarboxylation of α,β-unsaturated esters with CO2.

Scheme 36: Proposed reaction mechanism for the Rh-catalyzed hydrocarboxylation of C–C double bonds with CO2.

Scheme 37: Visible-light-driven hydrocarboxylation with CO2.

Scheme 38: Visible-light-driven Rh-catalyzed hydrocarboxylation of C–C double bonds with CO2.

Scheme 39: Optimization of reaction conditions on the Rh-catalyzed [2 + 2 + 2] cycloaddition of diyne 42a and ...

Scheme 40: [2 + 2 + 2] Cycloaddition of diyne and CO2.

Scheme 41: Proposed reaction pathways for the Rh-catalyzed [2 + 2 + 2] cycloaddition of diyne and CO2.
Diazirine-functionalized mannosides for photoaffinity labeling: trouble with FimH
- Femke Beiroth,
- Tomas Koudelka,
- Thorsten Overath,
- Stefan D. Knight,
- Andreas Tholey and
- Thisbe K. Lindhorst
Beilstein J. Org. Chem. 2018, 14, 1890–1900, doi:10.3762/bjoc.14.163
- ][2][3][4]. Photoaffinity labeling requires a ligand equipped with a photolabile group, which can be converted into a highly reactive intermediate upon irradiation with light of an appropriate wavelength. This technique involves incubation of the photolabile ligand with the target protein (receptor

Graphical Abstract

Figure 1: Principle of photoaffinity labeling of proteins with diazirine derivatives. Photolabile ligands are...

Figure 2: FimH crystal structure (pdb code 1KLF) with docked p-nitrophenyl α-D-mannopyranoside (1, pNPMan). F...

Figure 3: Based on the structure of the known FimH ligands 1 and 2, three photolabile α-D-mannosides, 3–5, we...

Figure 4: Connolly representation of photolabile α-D-mannoside 3 in the closed gate (A, PDB code 1UWF) and op...

Scheme 1: Synthesis of photolabile α-D-mannosides 3 and 4. a) H2, Pd-C, methanol, rt, 6 h, 94%; b) HATU, DIPE...

Figure 5: ESIMS spectra of peptide M2 before (A) and after photoreaction (B) with mannoside 3.

Figure 6: Intact protein ESIMS spectra of FimHtr labeling with diazirine derivative 3 with (A) 50% acetonitri...
Synthesis of new tricyclic 5,6-dihydro-4H-benzo[b][1,2,4]triazolo[1,5-d][1,4]diazepine derivatives by [3+ + 2]-cycloaddition/rearrangement reactions
- Lin-bo Luan,
- Zi-jie Song,
- Zhi-ming Li and
- Quan-rui Wang
Beilstein J. Org. Chem. 2018, 14, 1826–1833, doi:10.3762/bjoc.14.155
- the synthesis with a proposed mechanism as outlined in Scheme 5 with the α-acetoxyphenylazo compound 8a serving as a model substrate. In this pathway, the reaction is initiated by AlCl3 coordination with the acetate moiety of 8a to generate the azocarbenium ion 14 as a reactive intermediate, which

Graphical Abstract

Figure 1: Examples of marketed pharmaceutical 1,2,4-triazolobenzodiazepines.

Scheme 1: Preparation of N-acylated 2,3-dihydro-4(1H)-quinolones 6.

Scheme 2: Synthesis of α-acetoxyazo compounds 8a–g. Reaction conditions: for synthesis of 8a: 7a (10.42 mmol)...

Scheme 3: Synthesis of tricyclic benzo[b][1,2,4]triazolo[1,5-d][1,4]diazepinium salts 10. Reaction conditions...

Scheme 4: Synthesis of N(1)-unsubstituted benzo[b][1,2,4]triazolo[1,5-d][1,4]diazepines 13. Reaction conditio...

Scheme 5: Mechanistic rationale for the [3+ + 2]-cycloaddition/rearrangement reaction.

Figure 2: Crystal structure of salt 10k. The displacement ellipsoids are drawn at the 30% probability level.

Figure 3: Crystal structure of the free base 13e. The displacement ellipsoids are drawn at the 30% probabilit...
Mannich base-connected syntheses mediated by ortho-quinone methides
- Petra Barta,
- Ferenc Fülöp and
- István Szatmári
Beilstein J. Org. Chem. 2018, 14, 560–575, doi:10.3762/bjoc.14.43
- indication that the application of this highly-reactive intermediate has made the modified Mannich reaction to be a hot topic again in organic chemistry. This review presents a wide range of applications including cycloadditions and the synthesis of bifunctional amino- or amidonaphthols that can later be
- be explained by the generation of an o-QM intermediate followed by the nucleophilic addition of the amine component, the reverse reaction with the corresponding nucleophile is also feasible. Mechanistically, the Mannich adduct generates an o-QM via the loss of an amine, then this reactive
- intermediate reacts with the nucleophile (dienophile) species in different reactions to form a wide range of heterocyclic compounds. Reactions with C=C dienophiles Reactions of o-QMs with different C=C dienophiles are listed in Table 3. Osyanin et al. reported the efficient reaction of quaternary ammonium salt

Graphical Abstract

Scheme 1: Formation of amidoalkylnaphthols 4 via o-QM intermediate 3.

Scheme 2: Asymmetric syntheses of triarylmethanes starting from diarylmethylamines.

Scheme 3: Proposed mechanism for the formation of 2,2-dialkyl-3-dialkylamino-2,3-dihydro-1H-naphtho[2,1-b]pyr...

Scheme 4: Cycloadditions of isoflavonoid-derived o-QMs and various dienophiles.

Scheme 5: [4 + 2] Cycloaddition reactions between aminonaphthols and cyclic amines.

Scheme 6: Brønsted acid-catalysed reaction between aza-o-QMs and 2- or 3-substituted indoles.

Scheme 7: Formation of 3-(α,α-diarylmethyl)indoles 52 in different synthetic pathways.

Scheme 8: Alkylation of o-QMs with N-, O- or S-nucleophiles.

Scheme 9: Formation of DNA linkers and o-QM mediated polymers.
Photocatalytic formation of carbon–sulfur bonds
- Alexander Wimmer and
- Burkhard König
Beilstein J. Org. Chem. 2018, 14, 54–83, doi:10.3762/bjoc.14.4
- form an aryl radical. This reactive intermediate reacts with the anion of the respective thiol to form the corresponding diaryl sulfide adducts. The reaction conditions tolerate functional groups like alcohols, esters, halides, the trifluoromethyl group and heterocyclic substrates like thiazoles or

Graphical Abstract

Scheme 1: General overview over the sulfur-based substrates and reactive intermediates that are discussed in ...

Scheme 2: Photoredox-catalyzed radical thiol–ene reaction, applying [Ru(bpz)3](PF6)2 as photocatalyst.

Scheme 3: Photoredox-catalyzed thiol–ene reaction of aliphatic thiols with alkenes enabled by aniline derivat...

Scheme 4: Photoredox-catalyzed radical thiol–ene reaction for the postfunctionalization of polymers (a) and n...

Scheme 5: Photoredox-catalyzed thiol–ene reaction enabled by bromotrichloromethane as redox additive.

Scheme 6: Photoredox-catalyzed preparation of β-ketosulfoxides with Eosin Y as organic dye as photoredox cata...

Scheme 7: Greaney’s photocatalytic radical thiol–ene reaction, applying TiO2 nanoparticles as photocatalyst.

Scheme 8: Fadeyi’s photocatalytic radical thiol–ene reaction, applying Bi2O3 as photocatalyst.

Scheme 9: Ananikov’s photocatalytic radical thiol-yne reaction, applying Eosin Y as photocatalyst.

Scheme 10: Organocatalytic visible-light photoinitiated thiol–ene coupling, applying phenylglyoxylic acid as o...

Scheme 11: Xia’s photoredox-catalyzed synthesis of 2,3-disubstituted benzothiophenes, applying 9-mesityl-10-me...

Scheme 12: Wang’s metal-free photoredox-catalyzed radical thiol–ene reaction, applying 9-mesityl-10-methylacri...

Scheme 13: Visible-light benzophenone-catalyzed metal- and oxidant-free radical thiol–ene reaction.

Scheme 14: Visible-light catalyzed C-3 sulfenylation of indole derivatives using Rose Bengal as organic dye.

Scheme 15: Photocatalyzed radical thiol–ene reaction and subsequent aerobic sulfide-oxidation with Rose Bengal...

Scheme 16: Photoredox-catalyzed synthesis of diaryl sulfides.

Scheme 17: Photocatalytic cross-coupling of aryl thiols with aryl diazonium salts, using Eosin Y as photoredox...

Scheme 18: Photocatalyzed cross-coupling of aryl diazonium salts with cysteines in batch and in a microphotore...

Scheme 19: Fu’s [Ir]-catalyzed photoredox arylation of aryl thiols with aryl halides.

Scheme 20: Fu’s photoredox-catalyzed difluoromethylation of aryl thiols.

Scheme 21: C–S cross-coupling of thiols with aryl iodides via [Ir]-photoredox and [Ni]-dual-catalysis.

Scheme 22: C–S cross-coupling of thiols with aryl bromides, applying 3,7-bis-(biphenyl-4-yl)-10-(1-naphthyl)ph...

Scheme 23: Collin’s photochemical dual-catalytic cross-coupling of thiols with bromoalkynes.

Scheme 24: Visible-light-promoted C–S cross-coupling via intermolecular electron donor–acceptor complex format...

Scheme 25: Li’s visible-light photoredox-catalyzed thiocyanation of indole derivatives with Rose Bengal as pho...

Scheme 26: Hajra’s visible-light photoredox-catalyzed thiocyanation of imidazoheterocycles with Eosin Y as pho...

Scheme 27: Wang’s photoredox-catalyzed thiocyanation reaction of indoles, applying heterogeneous TiO2/MoS2 nan...

Scheme 28: Yadav’s photoredox-catalyzed α-C(sp3)–H thiocyanation reaction for tertiary amines, applying Eosin ...

Scheme 29: Yadav’s photoredox-catalyzed synthesis of 5-aryl-2-imino-1,3-oxathiolanes.

Scheme 30: Yadav’s photoredox-catalyzed synthesis of 1,3-oxathiolane-2-thiones.

Scheme 31: Li’s photoredox catalysis for the preparation of 2-substituted benzothiazoles, applying [Ru(bpy)3](...

Scheme 32: Lei’s external oxidant-free synthesis of 2-substituted benzothiazoles by merging photoredox and tra...

Scheme 33: Metal-free photocatalyzed synthesis of 2-aminobenzothiazoles, applying Eosin Y as photocatalyst.

Scheme 34: Metal-free photocatalyzed synthesis of 1,3,4-thiadiazoles, using Eosin Y as photocatalyst.

Scheme 35: Visible-light photoredox-catalyzed preparation of benzothiophenes with Eosin Y.

Scheme 36: Visible-light-induced KOH/DMSO superbase-promoted preparation of benzothiophenes.

Scheme 37: Jacobi von Wangelin’s photocatalytic approach for the synthesis of aryl sulfides, applying Eosin Y ...

Scheme 38: Visible-light photosensitized α-C(sp3)–H thiolation of aliphatic ethers.

Scheme 39: Visible-light photocatalyzed cross-coupling of alkyl and aryl thiosulfates with aryl diazonium salt...

Scheme 40: Visible-light photocatalyzed, controllable sulfenylation and sulfoxidation with organic thiosulfate...

Scheme 41: Rastogi’s photoredox-catalyzed methylsulfoxidation of aryl diazonium salts, using [Ru(bpy)3]Cl2 as ...

Scheme 42: a) Visible-light metal-free Eosin Y-catalyzed procedure for the preparation of vinyl sulfones from ...

Scheme 43: Visible-light photocatalyzed cross-coupling of sodium sulfinates with secondary enamides.

Scheme 44: Wang’s photocatalyzed oxidative cyclization of phenyl propiolates with sulfinic acids, applying Eos...

Scheme 45: Lei’s sacrificial oxidant-free synthesis of allyl sulfones by merging photoredox and transition met...

Scheme 46: Photocatalyzed Markovnikov-selective radical/radical cross-coupling of aryl sulfinic acids and term...

Scheme 47: Visible-light Eosin Y induced cross-coupling of aryl sulfinic acids and styrene derivatives, afford...

Scheme 48: Photoredox-catalyzed bicyclization of 1,7-enynes with sulfinic acids, applying Eosin Y as photocata...

Scheme 49: Visible-light-accelerated C–H-sulfinylation of arenes and heteroarenes.

Scheme 50: Visible-light photoredox-catalyzed β-selenosulfonylation of electron-rich olefins, applying [Ru(bpy)...

Scheme 51: Photocatalyzed preparation of β-chlorosulfones from the respective olefins and p-toluenesulfonyl ch...

Scheme 52: a) Photocatalyzed preparation of β-amidovinyl sulfones from sulfonyl chlorides. b) Preparation of β...

Scheme 53: Visible-light photocatalyzed sulfonylation of aliphatic tertiary amines, applying [Ru(bpy)3](PF6)2 ...

Scheme 54: Reiser’s visible-light photoredox-catalyzed preparation of β-hydroxysulfones from sulfonyl chloride...

Scheme 55: a) Sun’s visible-light-catalyzed approach for the preparation of isoquinolinonediones, applying [fac...

Scheme 56: Visible-light photocatalyzed sulfonylation/cyclization of vinyl azides, applying [Ru(bpy)3]Cl2 as p...

Scheme 57: Visible-light photocatalyzed procedure for the formation of β-ketosulfones from aryl sulfonyl chlor...

Scheme 58: Zheng’s method for the sulfenylation of indole derivatives, applying sulfonyl chlorides via visible...

Scheme 59: Cai’s visible-light induced synthesis of β-ketosulfones from sulfonyl hydrazines and alkynes.

Scheme 60: Photoredox-catalyzed approach for the preparation of vinyl sulfones from sulfonyl hydrazines and ci...

Scheme 61: Jacobi von Wangelin’s visible-light photocatalyzed chlorosulfonylation of anilines.

Scheme 62: Three-component photoredox-catalyzed synthesis of N-amino sulfonamides, applying PDI as organic dye....

Scheme 63: Visible-light induced preparation of complex sulfones from oximes, silyl enol ethers and SO2.
Preactivation-based chemoselective glycosylations: A powerful strategy for oligosaccharide assembly
- Weizhun Yang,
- Bo Yang,
- Sherif Ramadan and
- Xuefei Huang
Beilstein J. Org. Chem. 2017, 13, 2094–2114, doi:10.3762/bjoc.13.207
- . In comparison, the preactivation-based iterative glycosylation is unique, where a glycosyl donor is preactivated in the absence of an acceptor to produce a reactive intermediate (Scheme 1b) [18][19][20][21]. Upon complete donor activation, the acceptor is added to the reaction mixture, which
- drawbacks of the reactivity-based chemoselective glycosylation can be overcome through preactivation. Under the preactivation protocol, a thioglycosyl donor is activated in the absence of an acceptor to form a reactive intermediate (Scheme 13). Upon complete donor activation, a thioglycosyl acceptor is

Graphical Abstract

Scheme 1: a) Traditional glycosylation typically employs the premixed approach with both the donor and the ac...

Scheme 2: Glycosylation of an unreactive substrate. Reagents and conditions: (a) Tf2O, −78 °C, CH2Cl2 (DCM), ...

Scheme 3: Bromoglycoside-mediated glycosylation.

Scheme 4: Glycosyl bromide-mediated selenoglycosyl donor-based iterative glycosylation. Reagents and conditio...

Scheme 5: Preactivation-based glycosylation using 2-pyridyl glycosyl donors.

Scheme 6: Chemoselective dehydrative glycosylation. Reagents and conditions: (a) Ph2SO, Tf2O, 2-chloropyridin...

Figure 1: Representative structures of products formed by the preactivation-based dehydrative glycosylation o...

Scheme 7: Possible mechanism for the dehydrative glycosylation. (a) Formation of diphenyl sulfide bis(triflat...

Scheme 8: Chemoselective iterative dehydrative glycosylation. Reagents and conditions: (a) Ph2SO, Tf2O, 2,4,6...

Scheme 9: Chemoselective iterative dehydrative glycosylation. Reagents and conditions: (a) Ph2SO, Tf2O, −40 °...

Scheme 10: Chemical synthesis of a hyaluronic acid (HA) trimer 47. Reagents and conditions: (a) Ph2SO, TTBP, CH...

Figure 2: Retrosynthetic analysis of pentasaccharide 48.

Scheme 11: Effects of anomeric leaving groups on glycosylation outcomes. Reagents and conditions: (a) Ph2SO, Tf...

Scheme 12: Reactivity-based one-pot chemoselective glycosylation.

Scheme 13: Preactivation-based iterative glycosylation of thioglycosides.

Scheme 14: BSP/Tf2O promoted synthesis of 75.

Scheme 15: Proposed mechanism for preactivation-based glycosylation strategy.

Figure 3: The preactivations of glycosyl donors 83, 85 and 87 were investigated by low temperature NMR, which...

Scheme 16: The more electron-rich glycosyl donor 91 gave a higher glycosylation yield than the glycosyl donor ...

Scheme 17: Comparison of the BSP/Tf2O and p-TolSCl/AgOTf promoter systems in facilitating the preactivation-ba...

Scheme 18: One-pot synthesis of Globo-H hexasaccharide 105 using building blocks 101, 102, 103 and 104.

Scheme 19: Synthesis of (a) oligosaccharides 109–113 towards (b) 30-mer galactan 115. Reagents and conditions:...

Figure 4: Structure of mycobacterial arabinogalactan 116.

Figure 5: Representative complex glycans from glycolipid family synthesized by the preactivation-based thiogl...

Figure 6: Representative microbial and mammalian oligosaccharides synthesized by the preactivation-based thio...

Figure 7: Some representative mammalian oligosaccharides synthesized by the preactivation-based thioglycoside...

Figure 8: Preparation of a heparan sulfate oligosaccharides library.

Scheme 20: Synthesis of oligo-glucosamines through electrochemical promoted preactivation-based thioglycoside ...

Scheme 21: Synthesis of 2-deoxyglucosides through preactivation. Reagents and conditions: a) AgOTf, p-TolSCl, ...

Scheme 22: Synthesis of tetrasaccharide 153. Reagents and conditions: (a) AgOTf, p-TolSCl, CH2Cl2, −78 °C; the...

Scheme 23: Aglycon transfer from a thioglycosyl acceptor to an activated donor can occur during preactivation-...
Mechanochemical synthesis of small organic molecules
- Tapas Kumar Achar,
- Anima Bose and
- Prasenjit Mal
Beilstein J. Org. Chem. 2017, 13, 1907–1931, doi:10.3762/bjoc.13.186
- anilines proceed through the formation of unisolable reactive intermediate, aryl N-thiocarbamoylbenzotriazole, which rapidly decomposes to the corresponding isothiocyanate in organic solvent [189]. The Štrukil and Friščić group successfully demonstrated the formation of aryl-N-thiocarbamoylbenzotriazole
- under the LAG (liquid-assisted grinding) synthesis (Scheme 58) [190]. Initially, in situ monitoring of mechanochemical thiocarbamoylation suggests the formation of reactive intermediate which gradually disappears with the formation of thiocarbamoylated product. Furthermore isolation and spectroscopic

Graphical Abstract

Scheme 1: Mechanochemical aldol condensation reactions [48].

Scheme 2: Enantioselective organocatalyzed aldol reactions under mechanomilling. a) Based on binam-(S)-prolin...

Scheme 3: Mechanochemical Michael reaction [51].

Scheme 4: Mechanochemical organocatalytic asymmetric Michael reaction [52].

Scheme 5: Mechanochemical Morita–Baylis–Hillman (MBH) reaction [53].

Scheme 6: Mechanochemical Wittig reactions [55].

Scheme 7: Mechanochemical Suzuki reaction [56].

Scheme 8: Mechanochemical Suzuki–Miyaura coupling by LAG [57].

Scheme 9: Mechanochemical Heck reaction [59].

Scheme 10: a) Sonogashira coupling under milling conditions. b) The representative example of a double Sonogas...

Scheme 11: Copper-catalyzed CDC reaction under mechanomilling [67].

Scheme 12: Asymmetric alkynylation of prochiral sp3 C–H bonds via CDC [68].

Scheme 13: Fe(III)-catalyzed CDC coupling of 3-benzylindoles [69].

Scheme 14: Mechanochemical synthesis of 3-vinylindoles and β,β-diindolylpropionates [70].

Scheme 15: Mechanochemical C–N bond construction using anilines and arylboronic acids [78].

Scheme 16: Mechanochemical amidation reaction from aromatic aldehydes and N-chloramine [79].

Scheme 17: Mechanochemical CDC between benzaldehydes and benzyl amines [81].

Scheme 18: Mechanochemical protection of -NH2 and -COOH group of amino acids [85].

Scheme 19: Mechanochemical Ritter reaction [87].

Scheme 20: Mechanochemical synthesis of dialkyl carbonates [90].

Scheme 21: Mechanochemical transesterification reaction using basic Al2O3 [91].

Scheme 22: Mechanochemical carbamate synthesis [92].

Scheme 23: Mechanochemical bromination reaction using NaBr and oxone [96].

Scheme 24: Mechanochemical aryl halogenation reactions using NaX and oxone [97].

Scheme 25: Mechanochemical halogenation reaction of electron-rich arenes [88,98].

Scheme 26: Mechanochemical aryl halogenation reaction using trihaloisocyanuric acids [100].

Scheme 27: Mechanochemical fluorination reaction by LAG method [102].

Scheme 28: Mechanochemical Ugi reaction [116].

Scheme 29: Mechanochemical Passerine reaction [116].

Scheme 30: Mechanochemical synthesis of α-aminonitriles [120].

Scheme 31: Mechanochemical Hantzsch pyrrole synthesis [121].

Scheme 32: Mechanochemical Biginelli reaction by subcomponent synthesis approach [133].

Scheme 33: Mechanochemical asymmetric multicomponent reaction[134].

Scheme 34: Mechanochemical Paal–Knorr pyrrole synthesis [142].

Scheme 35: Mechanochemical synthesis of benzothiazole using ZnO nano particles [146].

Scheme 36: Mechanochemical synthesis of 1,2-di-substituted benzimidazoles [149].

Scheme 37: Mechanochemical click reaction using an alumina-supported Cu-catalyst [152].

Scheme 38: Mechanochemical click reaction using copper vial [155].

Scheme 39: Mechanochemical indole synthesis [157].

Scheme 40: Mechanochemical synthesis of chromene [158].

Scheme 41: Mechanochemical synthesis of azacenes [169].

Scheme 42: Mechanochemical oxidative C-P bond formation [170].

Scheme 43: Mechanochemical C–chalcogen bond formation [171].

Scheme 44: Solvent-free synthesis of an organometallic complex.

Scheme 45: Selective examples of mechano-synthesis of organometallic complexes. a) Halogenation reaction of Re...

Scheme 46: Mechanochemical activation of C–H bond of unsymmetrical azobenzene [178].

Scheme 47: Mechanochemical synthesis of organometallic pincer complex [179].

Scheme 48: Mechanochemical synthesis of tris(allyl)aluminum complex [180].

Scheme 49: Mechanochemical Ru-catalyzed olefin metathesis reaction [181].

Scheme 50: Rhodium(III)-catalyzed C–H bond functionalization under mechanochemical conditions [182].

Scheme 51: Mechanochemical Csp2–H bond amidation using Ir(III) catalyst [183].

Scheme 52: Mechanochemical Rh-catalyzed Csp2–X bond formation [184].

Scheme 53: Mechanochemical Pd-catalyzed C–H activation [185].

Scheme 54: Mechanochemical Csp2–H bond amidation using Rh catalyst.

Scheme 55: Mechanochemical synthesis of indoles using Rh catalyst [187].

Scheme 56: Mizoroki–Heck reaction of aminoacrylates with aryl halide in a ball-mill [58].

Scheme 57: IBX under mechanomilling conditions [8].

Scheme 58: Thiocarbamoylation of anilines; trapping of reactive aryl-N-thiocarbamoylbenzotriazole intermediate...






